Cytiva – Moving to large scale AAV manufacturing: Spotlight on producer cell lines

Facts about AAV producer cell lines
Gene therapies hold almost unlimited promise to treat diseases that have a genetic component. Several gene therapies are commercially available, with adeno-associated virus (AAV) emerging as a popular viral vector for gene delivery. Because of speed and available technology, approved therapies were produced using triple-plasmid transient transfection and adherent cells. Good titers of infectious virus with sufficient quality have been achieved with the ability to scale up to larger adherent cell culture processes and even to suspension culture in stirred-tank bioreactors. Thus, transient transfection is effective, but many scientists making AAV envision a future where producer cell lines will replace transient transfection in large-scale scenarios for prevalent and even ultimately common diseases.
Here's our list of top facts about AAV producer cell lines for gene therapies.
Fact: Definitions of AAV producer cell lines vary
Producer cell lines are genetically engineered to stably produce the target of interest and are designed for use in large-scale manufacturing platforms. They're well-established in monoclonal antibody (mAb) production, where they have been used at scales larger than 2000 L and have enabled other improvements in upstream processing to enhance production in smaller volumes. One example is process intensification using perfusion processing mode. A true AAV producer cell line doesn't require helper virus or any transfection. That's because all required genetic elements are stably integrated into the host genome. It's important to note that production cell lines are not the same as producer cell lines. Production cell lines simply refer to any cell lines producing viral vectors – whether in a transient or a stable system.
Fact: Developing a true AAV producer cell line takes skill
The difficulties in creating an efficient proprietary system are primarily rooted in the complexity of cellular biology. A true AAV producer cell line requires incorporating four elements — Rep, Helper, Cap, and the gene of interest (GOI) — into the host genome. These must be skillfully engineered and precisely balanced to support robust cell growth, viral titer, and quality.
At Cytiva, we have developed two separate alpha cell line platforms: a clonal HEK293 alpha cell line and a clonal CAP™ alpha cell line that is derived from human amniocytes. Both alpha cell lines have Rep and Helper functions already stably integrated into their genome. We use single-cell deposition and high-throughput screening technologies for single-cell cloning of the producer pool, from which we then select the best producer single-cell clones that give high productivity, packaging efficiency, and product quality. Figure 1 shows how we develop our producer cell lines.
Another significant challenge in developing an effective tailored cell line is maintaining cell adaptability and robustness throughout the entire process, including scale-up. To achieve this, it's important to optimize cell culture conditions to maintain cell viability and growth while preventing aggregation. These conditions can be achieved through extensive media and supplementation testing.
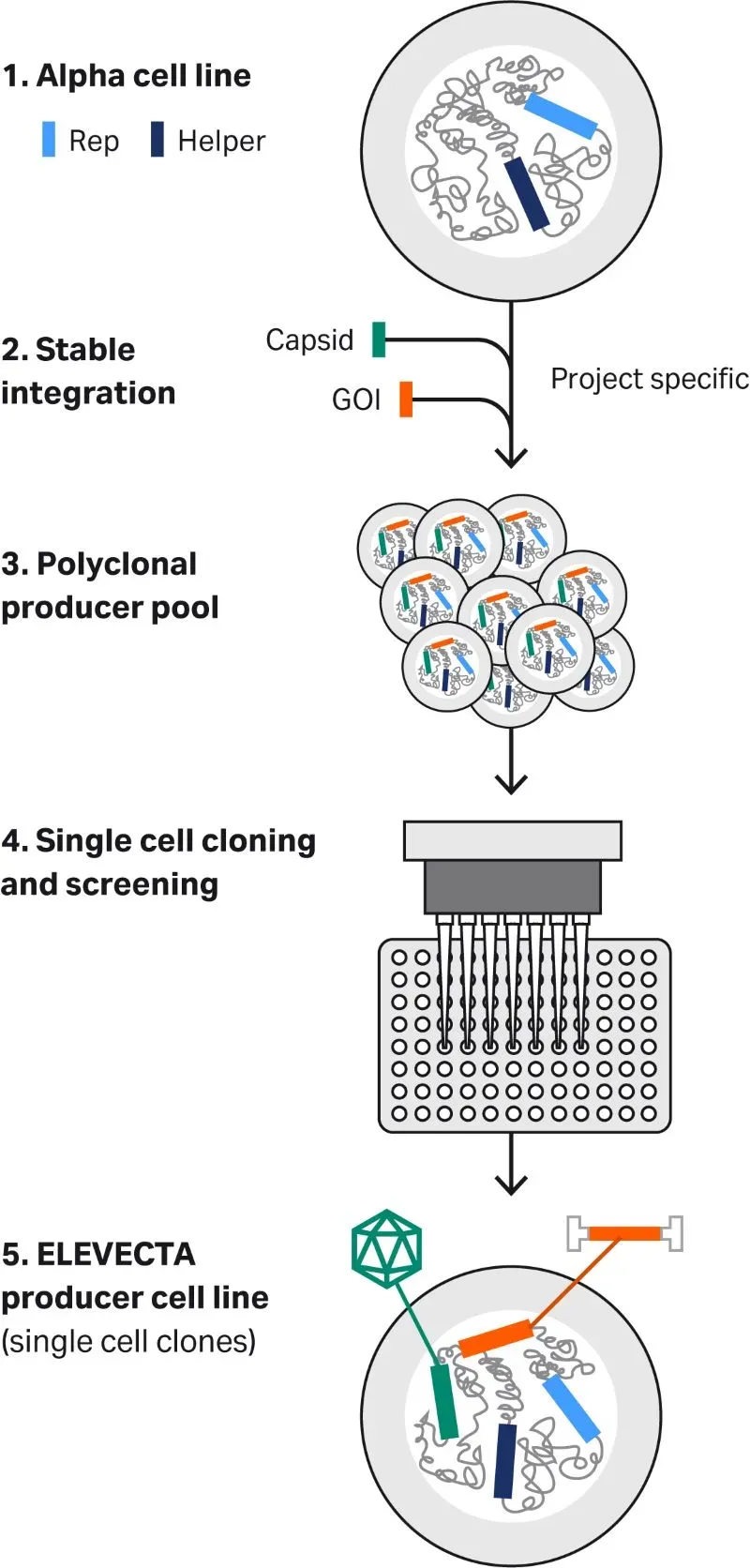
Fig 1. ELEVECTA™ producer cell line development process.
Fact: AAV producer cell lines offer several process benefits
First, a true AAV producer cell line simplifies production by eliminating the need for transient transfection and helper virus. It takes just one step to start production by adding an inducing agent to the production bioreactor to initiate production. Next, such a cell line helps to minimize batch-to-batch variation, particularly when single-cell cloning is employed. There's no variation from materials such as transfection reagent and plasmids, as they're not used. The stable, inducible production is easy to replicate, with cells originating from a working cell bank (WCB). Producer cell lines support manufacturing scales in the thousands of liters, where transient transfection will be challenging to scale and will be very expensive. Finally, this production option supports a robust supply chain, as it's not necessary to source large quantities of high-quality plasmids and transfection reagent.
Fact: They support lower cost of goods manufactured (COGm)
Plasmids and transfection reagent represent large expenses when producing an AAV batch. This cost goes up as the scale increases, because more of each material is needed. Eliminating these expensive components has the potential to lower the costs of making a batch.
In an exercise to better understand how stable cell lines can ultimately contribute to the cost of manufacturing AAV, we employ cost modeling tools. We recently generated some data comparing transfection with a producer cell line for different AAV throughputs per year. Cost modeling data suggests that producer cell lines have the potential to bring economical interest depending on multiple factors including yearly AAV throughput required, viral titer, full-empty ratio, DNA per cell ratio, etc. The cost reduction mainly comes from eliminating the need for plasmid DNA and transfection reagent, which together typically represent approximately 40% — depending on process conditions — of the costs to manufacture AAV.
Take the next step
Want to know more about stable producer cell lines?
Watch the webinar
or connect directly with our specialists!
About Cytiva
At Cytiva, our mission is to advance and accelerate the development of therapeutics. With 15 000 associates in more than 40 countries, we’re driven to use our expertise and talent to achieve better flexibility, capacity, and efficiency for our customers. Our broad and deep portfolio of tools and technologies, global scale, and best-in-class service provides critical support from discovery to delivery, for customers spanning researchers, emerging biotech, large-scale biopharma and contract manufacturers. Learn more at cytiva.com


