Combining FAK Inhibition with ADCs to Break Tumor Barriers and Boost Efficacy
Antibody–drug conjugates (ADCs) have emerged as “biological missiles” against cancer, achieving notable successes in multiple tumor types. For example, HER2-targeted ADCs like trastuzumab deruxtecan (Enhertu) and TROP2-targeted ADCs like sacituzumab govitecan (Trodelvy) have demonstrated significant therapeutic potential pmc.ncbi.nlm.nih.gov. These agents deliver potent cytotoxic payloads directly to antigen-expressing tumor cells, and recent clinical trials have expanded ADC use even in traditionally hard-to-treat settings (including HER2-low tumors ) pmc.ncbi.nlm.nih.govpmc.ncbi.nlm.nih.gov. However, despite these advances, a subset of patients remain refractory to ADC therapy, showing only minimal tumor responses pmc.ncbi.nlm.nih.gov. This gap highlights a critical challenge: effective ADC delivery in solid tumors. Indeed, intratumoral distribution of ADCs – how deeply and uniformly they penetrate the tumor – plays a major role in determining efficacy pmc.ncbi.nlm.nih.gov. In many solid tumors, particularly those with dense stromal tissue, the large ADC molecules struggle to reach all cancer cells.
One major culprit behind poor ADC penetration is the tumor microenvironment, especially the presence of cancer-associated fibroblasts (CAFs) and fibrotic stroma. These stromal cells produce collagen and extracellular matrix that can act as a physical barrier around tumor nests. In high-stroma tumors, ADCs often accumulate in perivascular regions and fail to infiltrate beyond the fibrotic shell. As one recent conference presentation put it, “high-stroma tumors remain highly challenging due to poor penetration of ADCs across the fibrotic barrier” delta.larvol.com. In other words, even a well-designed ADC can be “held at arm’s length” by the tumor’s defensive stroma, preventing the drug from reaching its targets. Overcoming this barrier is therefore a key strategy to optimize ADC efficacy and convert non-responders into responders pmc.ncbi.nlm.nih.gov.
Enter focal adhesion kinase (FAK) – a non-receptor tyrosine kinase that is overexpressed and hyperactivated in many tumors, particularly within the tumor stroma pubmed.ncbi.nlm.nih.gov. AK is a critical signaling hub for cell adhesion, migration, and survival, linking integrin signaling from the extracellular matrix to tumor cell behavior. Notably, FAK activity in the tumor microenvironment has been linked to stromal fibrosis, poor perfusion, and immune suppression pubmed.ncbi.nlm.nih.gov. High FAK phosphorylation is common in desmoplastic cancers (e.g. pancreatic, ovarian) and correlates with worse patient survival pubmed.ncbi.nlm.nih.gov. These findings have spurred the development of multiple FAK inhibitors (small-molecule, oral agents) aimed at targeting both tumor cells and stromal cells. To date, numerous FAK inhibitors (including defactinib, IN10018, GSK2256098, and others) have shown anti-tumor activity in preclinical studies and have entered clinical trials delta.larvol.com. Importantly, combination approaches are a focal point of FAK inhibitor development, given FAK’s role in resistance to chemo, immunotherapy, and other treatments delta.larvol.com. One promising combination strategy – and the focus of this report – is pairing FAK inhibitors with ADCs to remodel the tumor microenvironment and thereby improve ADC uptake and anti-cancer responses.
In the sections below, we discuss the mechanistic rationale for combining FAK inhibition with ADC therapy, review recent preclinical and emerging clinical evidence supporting this approach, include insights from leaders in the field, and outline strategic implications for drug development. The goal is to provide a data-driven, strategic overview for senior R&D and scientific leadership on how this combination could enhance therapeutic outcomes in oncology.
Mechanistic Rationale: Remodeling the TME to Enhance ADC Delivery
Schematic illustration of how FAK inhibition can break down the fibrotic tumor barrier to enhance ADC penetration. (Left) In ADC monotherapy, abundant cancer-associated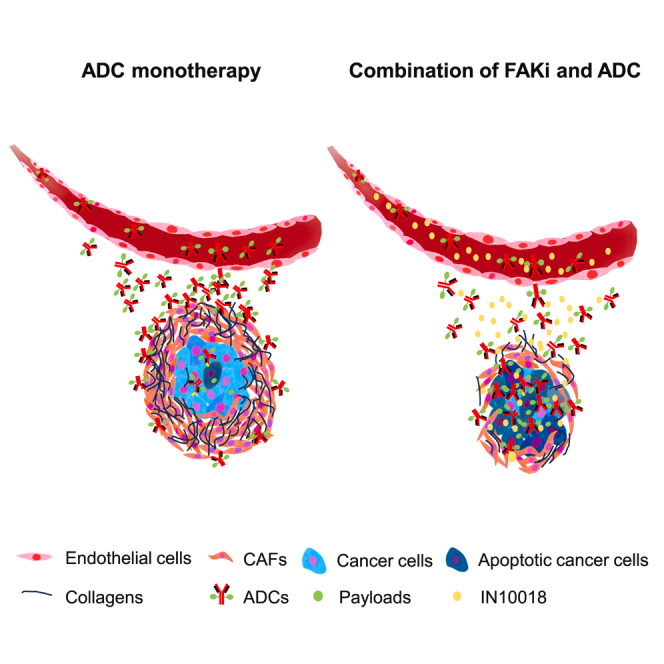 fibroblasts (CAFs) and collagen in the tumor microenvironment create a dense stroma (pink and black matrix) that limits the distribution of ADC molecules (green ⧫ with red antibodies) from blood vessels. (Right) With the addition of a FAK inhibitor (yellow ●), the fibrotic stroma is reduced, allowing deeper ADC penetration into the tumor, resulting in greater cancer cell kill (blue cells turning apoptotic).
fibroblasts (CAFs) and collagen in the tumor microenvironment create a dense stroma (pink and black matrix) that limits the distribution of ADC molecules (green ⧫ with red antibodies) from blood vessels. (Right) With the addition of a FAK inhibitor (yellow ●), the fibrotic stroma is reduced, allowing deeper ADC penetration into the tumor, resulting in greater cancer cell kill (blue cells turning apoptotic).
In solid tumors with dense stroma, FAK acts as a linchpin of the physical barrier that impedes drug delivery. Activated by integrins and growth factors, FAK in CAFs drives their proliferation and promotes deposition of collagen and extracellular matrix. Tumors essentially “hijack” normal fibroblasts, transforming them into FAK-activated CAFs that fortify the tumor’s defenses pmc.ncbi.nlm.nih.gov. The result is a fibrotic capsule around tumor cell clusters, elevated interstitial fluid pressure, and compressed blood vessels – all factors that impair the perfusion and tissue uptake of large therapeutic molecules like ADCs pmc.ncbi.nlm.nih.govdelta.larvol.com. This mechanism has been clearly demonstrated in recent studies: excessive CAFs were shown to form a barrier that dampens ADC uptake and efficacy in tumor models pmc.ncbi.nlm.nih.gov. Essentially, even if tumor cells express the target antigen and the ADC is potent, a heavy stromal presence can prevent adequate drug from reaching those cells, leading to treatment resistance pmc.ncbi.nlm.nih.gov.
Inhibiting FAK can reverse this process. By blocking FAK signaling, we can disrupt the cross-talk that sustains and activates CAFs. FAK inhibitors cause the collapse of the stromal barrier: CAF proliferation is reduced and the existing fibrotic matrix breaks downpmc.ncbi.nlm.nih.gov. In practical terms, FAK inhibition “softens” the tumor tissue, lowering interstitial pressure and increasing tumor vessel perfusion and permeability. With the barrier diminished, therapeutic antibodies and ADCs can penetrate deeper into the tumor mass. Experimental evidence shows that a FAK inhibitor effectively diminished the CAF-rich stroma and enhanced the intratumoral uptake of ADCs in preclinical models pmc.ncbi.nlm.nih.gov. Notably, this improved delivery was observed irrespective of the ADC’s target antigen, indicating a broad microenvironmental effect pmc.ncbi.nlm.nih.gov. Figure 1 above illustrates this concept: under FAK inhibition, previously “walled off” tumor cells become exposed to incoming ADC molecules, which can then bind to cancer cells and release their cytotoxic payload (represented as green and yellow dots in the schematic).
Mechanistically, FAK inhibition has multiple pro-perfusion and pro-infiltration effects. Fibrosis is reduced as collagen production drops, and enzymatic degradation may even be upregulated for existing matrix delta.larvol.com. There is also evidence that tumor hypoxia decreases when FAK is blocked, as seen with FAK inhibitors mitigating hypoxia and fibrosis in pancreatic cancer models pmc.ncbi.nlm.nih.govpmc.ncbi.nlm.nih.gov. This suggests blood flow is improving, which would further aid the distribution of drugs like ADCs. In addition, by reprogramming the stroma, FAK inhibition can indirectly modulate immune cell traffic: for instance, relieving physical barriers allows better infiltration of T cells and macrophages pmc.ncbi.nlm.nih.govpmc.ncbi.nlm.nih.gov. While the immune angle is a secondary benefit in the context of ADC delivery, it could enhance the overall anti-tumor response (especially since some ADCs can induce immunogenic cell death).
Another important discovery is that FAK inhibition may also improve the safety and therapeutic window of ADCs. Certain ADCs, such as Enhertu (which carries a topoisomerase inhibitor payload), are known to cause off-tumor effects like interstitial lung disease (ILD) in a minority of patients, thought to be related to an inflammatory or fibroblast activation in lung tissue. Intriguingly, preclinical research found that FAK activation is involved in the pathogenesis of ADC-induced pneumonitis, and using a FAK inhibitor was effective in preventing or treating ADC-induced lung toxicity in animal models en.inxmed.com. By reducing aberrant fibroblast activity in normal tissues as well, FAK inhibitors might protect against dose-limiting toxicities (like ILD) that currently constrain ADC dosing. As Dr. Zaiqi Wang (founder and CEO of InxMed) observed, “By increasing ADCs’ local exposure and reducing interstitial pneumonitis, FAK inhibition has the potential to significantly improve the therapeutic window” of ADC treatments en.inxmed.comen.inxmed.com. This dual mechanistic benefit – enhanced tumor kill and reduced systemic toxicity – makes the case for FAK inhibitors as ideal partners to ADC therapy.
In summary, the mechanistic rationale for combining a FAK inhibitor with an ADC is compelling: FAK blockade attacks the tumor’s protective fortress, enabling the ADC to flood in and do its job more effectively. This approach directly addresses one of the key biological hurdles in solid tumor treatment, shifting the odds in favor of the drug and the patient. We next examine how this rationale has translated into tangible results in preclinical studies, and how it is beginning to be tested in the clinic.
Preclinical Evidence: Enhanced Tumor Uptake and Efficacy with FAK+ADC
The synergy between FAK inhibition and ADC therapy is not just theoretical – it has been borne out by recent preclinical studies. In late 2024, researchers reported a series of in vivo experiments in which a small-molecule FAK inhibitor was combined with multiple ADCs targeting different tumor antigens pmc.ncbi.nlm.nih.gov. The study used models characterized by abundant CAF content to rigorously test the hypothesis that breaking the stromal barrier would improve ADC performance. The results were striking: co-treatment with a FAK inhibitor and an ADC consistently outperformed either treatment alone, across several tumor models pmc.ncbi.nlm.nih.gov. In HER2-positive cancer models (analogous to breast or gastric cancers), the addition of a FAK inhibitor significantly increased the tumor uptake of a HER2-targeted ADC and led to deeper and more durable tumor regression than the ADC alone pmc.ncbi.nlm.nih.gov. Similarly, in models using a TROP2-targeted ADC (relevant to triple-negative breast and other epithelial cancers), the combination therapy produced superior anti-tumor outcomes compared to monotherapy, overcoming the resistance that was observed with the ADC by itself pmc.ncbi.nlm.nih.gov.
Notably, these improvements were achieved without needing to alter the ADC molecule – it was the same drug performing better, simply because the microenvironment had been “primed” by FAK inhibition. Tumor tissue analyses confirmed the mechanism: combination-treated tumors showed fewer active CAFs and reduced fibrosis, along with higher concentrations of ADC and its released payload within the tumor mass pmc.ncbi.nlm.nih.gov. In essence, the FAK inhibitor transformed the tumors from a protected sanctuary into vulnerable targets, and the ADC was able to penetrate and destroy cancer cells that previously escaped its reach. These findings were presented at the 2024 AACR conference and have since been published, providing compelling evidence that targeting FAK “improves the tumor uptake of ADCs” and thereby strengthens anti-cancer responses pmc.ncbi.nlm.nih.gov. As summarized in one conference talk: “FAK inhibitor [treatment] is able to break [the] fibrotic barrier, consequently enhancing tissue penetration of ADC, [and] demonstrated significant synergy in vivo with multiple ADC molecules, especially in high-stroma tumor models.” delta.larvol.com.
It’s worth highlighting that this synergy was observed with different ADC payloads and targets, implying a broad utility. Whether the ADC was delivering a microtubule inhibitor or a topoisomerase inhibitor, in each case the FAK blocker increased its effectiveness, which bodes well for applying this combination across various ADC platforms. Another interesting observation from these studies was that FAK inhibition alone (without ADC) did not necessarily shrink tumors dramatically – its primary role was to modulate the microenvironment. But when paired with ADCs, the tumor growth inhibition was dramatically amplified, far beyond additive effects pmc.ncbi.nlm.nih.gov. This suggests a true synergy: the FAK inhibitor “flips a switch” that allows the ADC to reach more tumor cells, and the ADC then exerts its lethal effect on those cells with greater coverage. The outcome in animal models was not only slower tumor growth but in some cases complete tumor regressions where monotherapies had failed delta.larvol.com. These are the kinds of preclinical results that get translated into combination trial proposals, as they indicate a meaningful therapeutic advantage.
Beyond efficacy, the preclinical data also hinted at the combination’s potential to mitigate toxicity. We discussed earlier how FAK inhibition reduced ADC-related lung injury in animal models en.inxmed.com. This finding means that mice receiving the combo could tolerate continued treatment better than those on ADC alone, which ultimately allowed more dosing and contributed to better tumor control. For example, if an ADC’s dose would normally be cut or paused due to signs of lung toxicity, the presence of the FAK inhibitor allowed the full intended dose intensity to be delivered safely en.inxmed.com. This protection effect is a unique and unexpected advantage of the combo, essentially expanding the therapeutic window. From an efficacy standpoint, it ensures that the ADC can be used at its optimal dose for a longer duration, maximizing tumor kill. From a safety standpoint, it could be life-saving for patients, as it addresses one of the serious side effects that has accompanied some of the most potent ADCs.
In summary, the preclinical evidence strongly supports the FAK+ADC strategy. By simultaneously weakening the tumor’s defenses and attacking the tumor cells, the combination produced deeper responses in multiple models. These findings have provided the rationale to move into clinical testing, and they have caught the attention of both researchers and industry leaders. In the next section, we will discuss the early clinical developments in this area and include commentary from experts who are leading the charge in bringing FAK–ADC combinations to patients.
Expert Insights and Emerging Clinical Developments
The promising data from the lab have spurred action in the clinic. As of 2025, forward-thinking oncology teams are initiating clinical trials to evaluate FAK inhibitor plus ADC combinations. One notable frontrunner is InxMed, the company behind the FAK inhibitor IN10018 (also known as ifebemtinib). InxMed’s leadership has been vocal about the potential of this approach. Dr. Zaiqi Wang, CEO of InxMed, stated in a recent press release: “Ifebemtinib could become an ideal combo partner for ADCs, leading to revolutionary advancements in cancer therapy.” en.inxmed.com. This bold statement reflects the high expectations set by the preclinical results. Wang emphasized that by increasing local ADC exposure and reducing toxicity, FAK inhibition can significantly widen the therapeutic index of ADCs, which in turn could translate to better patient outcomes en.inxmed.com. Such commentary from industry leaders underscores a growing consensus that integrating tumor microenvironment modulators (like FAK inhibitors) is a key next step in advancing ADC therapy.
Translational efforts are well underway. In late 2024, InxMed announced it is submitting an IND (Investigational New Drug application) in China to begin clinical trials combining ifebemtinib with an ADC en.inxmed.com. While the specific ADC target hasn’t been publicly named, the company has hinted at collaborations with multiple ADC developers to explore combinations across different tumor types en.inxmed.com. This indicates a broad strategy to test the concept in various settings, potentially starting with tumor types known to have high stromal content or where current-generation ADCs have shown suboptimal results. For instance, triple-negative breast cancer, certain lung cancers, and pancreatic cancer could be prime candidates, given their desmoplastic stroma and need for improved therapies. The first-in-human studies will likely assess safety (making sure that adding the FAK inhibitor doesn’t introduce unexpected toxicities) and seek any early signs of improved anti-tumor activity (such as higher response rates or longer duration of response compared to historical data for the ADC alone).
It is important to note that, to date no FAK inhibitor has received full regulatory approval as a standalone therapy pmc.ncbi.nlm.nih.gov. All current FAK inhibitors are investigational, in phase I–II trials for various cancers. This means that the FAK+ADC combo strategy is truly at the cutting edge – it involves two advanced modalities, one of which is still experimental. However, there have been encouraging signals from clinical studies of FAK inhibitors in combination with other treatments. For example, ifebemtinib itself has shown impressive synergy with a KRAS-targeted therapy (a different combination) in early trials, yielding a 90% objective response rate in a KRAS-mutant lung cancer cohort delta.larvol.com. More relevantly, combining ifebemtinib with a liposomal chemotherapy (pegylated doxorubicin) in heavily pretreated ovarian cancer patients achieved an objective response rate of 56.7% and disease control in 86.7% of patients pmc.ncbi.nlm.nih.gov. This outcome, far above typical single-agent chemo results in such resistant cancer, earned ifebemtinib a Breakthrough Therapy designation in China for that combination. Such clinical data boost confidence that FAK inhibitors can be safely added to other therapies and meaningfully improve outcomes. They pave the way for trying similar combinations with ADCs. Other companies and research groups are likewise watching this space. Multiple FAK inhibitors are in development by different sponsors (Verastem’s defactinib, Novartis’s PND1186, GlaxoSmithKline’s GSK2256098, etc.), and many big pharma companies have deep ADC pipelines. This creates opportunities for cross-collaboration: a company with a portfolio of ADCs could partner with a company developing a FAK inhibitor to test combinations neither could pursue alone. In fact, the interest in tumor microenvironment modulation is not limited to FAK – there are parallel efforts investigating TGF-β inhibitors, hyaluronidase enzymes, and other stroma-targeted agents in combination with various therapies. But FAK inhibition has an edge in that it affects multiple facets of the microenvironment (fibrosis, vascular normalization, immune modulation) with a single target pubmed.ncbi.nlm.nih.gov. As such, some experts have dubbed FAK a potential “master switch” for making cold, fibrotic tumors hot and penetrable. We expect to see scientific conference presentations and publications in the next 1-2 years reporting initial clinical experiences with FAK+ADC combinations – detailing safety, optimal dosing, biomarker studies (e.g. measuring stromal density via imaging or biopsy), and any early efficacy signals.
One challenge will be patient selection and trial design. Not every tumor will need a FAK inhibitor; the combo will likely benefit those with a pronounced stromal barrier. Therefore, clinical trials may enrich for patients with high CAF or collagen signatures (for example, using biopsy analysis or MRI/PET imaging of fibrosis). This biomarker-driven approach could increase the chance of seeing a benefit in early trials. Additionally, the timing and sequence of administration will be explored – some preclinical evidence suggests that giving the FAK inhibitor a lead-in period to “open up” the tumor before administering the ADC dose might maximize uptake. Trials might test a schedule where patients receive the FAK inhibitor for a week, then add the ADC, versus starting both together, to see which yields better drug penetration (this could be assessed via imaging or tumor biopsies looking at ADC localization). From the patient perspective, adding an oral FAK inhibitor to an ADC regimen (which is typically IV infusions) is a manageable increase in treatment complexity, especially if it means improved outcomes. Safety monitoring will pay special attention to any overlapping toxicities; fortunately, the preclinical studies suggest no additive toxicity and even a reduction in certain ADC-related adverse events en.inxmed.com, but this will need confirmation in humans.
Overall, the expert sentiment is optimistic. There is a sense that we are at the brink of a new wave of ADC combination strategies. As one oncology researcher framed it, “Although ADC therapies are revolutionizing cancer treatment, we need to address the cases where tumors’ biology limits their efficacy. Targeting the stroma with agents like FAK inhibitors could be the key to reaching those ‘unreachable’ tumor cells.” The coming years will tell us just how much of a game-changer this approach will be in clinical oncology.
Strategic Implications for Drug Development
The convergence of ADC technology with tumor microenvironment modulation has significant strategic implications for oncology drug development. For senior R&D leaders and decision-makers, combining FAK inhibitors with ADCs represents both an opportunity and a paradigm shift in how we think about treating solid tumors. Below, we outline key strategic considerations and suggested actions:
- R&D Investment in Combination Therapies: Companies should prioritize preclinical and clinical development of FAK+ADC combinations in tumor types known for dense stroma and current unmet needs (e.g. triple-negative breast, pancreatic, ovarian, lung cancers). The strong preclinical proof-of-concept pmc.ncbi.nlm.nih.govdelta.larvol.com justifies allocating resources to these combination studies. This may involve funding translational research to identify optimal dosing schedules (sequencing vs. concurrent administration) and to develop biomarkers that predict which tumors will benefit most (such as a high CAF gene signature or elevated FAK activity in tumor biopsies). Early investment now could secure a leadership position in this emerging therapeutic area.
- Clinical Trial Design Considerations: When moving to clinical trials, design studies that can capture the added value of the combination. This might include innovative trial designs like adaptive umbrella trials where an ADC is tested alone vs. with one of several microenvironment modulators (with early dropping of ineffective arms). Ensure that trials incorporate biomarker endpoints – for instance, paired tumor biopsies before and after FAK inhibitor treatment to demonstrate increased ADC penetration or reduced fibrosis (this could be done via histology or imaging). Additionally, monitor safety closely, but leverage the potential protective effects of FAK inhibition; for example, track pulmonary function and biomarkers of ILD in trials with Enhertu to see if the FAK combo indeed reduces lung inflammation en.inxmed.com. If early-phase trials show even a hint of improved response rates or durability, be prepared to expedite to pivotal trials because the competitive window for first-in-class combo approval will narrow quickly if multiple players enter the field.
- Regulatory and Market Positioning: Engage with regulatory agencies early to discuss the combination strategy. Since neither the ADC nor the FAK inhibitor may be approved (depending on the assets in question), regulators will evaluate the combination’s risk/benefit holistically. Highlight the rationale that adding the FAK inhibitor could improve efficacy and possibly safety – a rare combo win-win – and support this with data (preclinical and any initial clinical). Agencies have shown willingness to grant Breakthrough Therapy designations when combo regimens show substantial improvement in serious conditions (as seen with ifebemtinib + chemo in ovarian cancer) pmc.ncbi.nlm.nih.gov. From a market perspective, a successful FAK+ADC combo could extend the patent life and marketability of an ADC franchise by moving it into new indications (e.g., tumors that were previously ADC-resistant). It can also differentiate an ADC in a crowded market: if two companies have similar ADCs, but one is able to pair it with a FAK inhibitor and show superior outcomes, that combination will capture clinician attention and likely market share. Therefore, strategize lifecycle management of ADC products with combination use in mind.
- Partnerships and Pipeline Synergies: Very few companies possess top-tier capabilities in both ADCs and TME-targeting small molecules. This opens the door for strategic partnerships. We are already seeing examples: InxMed, primarily a TME/FAK company, is establishing collaborations with multiple ADC companies to explore combos en.inxmed.com. Pharma and biotech leaders should identify potential partners to fill gaps – for instance, an ADC-focused biotech could partner with a company developing a FAK inhibitor to get early access to that asset for combination trials (and vice versa). Co-development deals or trial collaborations can accelerate the proof-of-concept in humans while sharing costs and risks. Additionally, consider in-licensing opportunities: some companies may choose to license a FAK inhibitor asset to combine with their in-house ADCs (or even acquire a smaller company outright if the FAK program is highly promising). Given that multiple FAK inhibitors are in mid-stage trials, there may be a chance to secure one that fits well with your portfolio. It’s also wise to look beyond FAK – the broader theme is “conditioning the tumor microenvironment” for ADC success. This could include evaluating other stromal or immune modulators in combination (e.g., angiotensin inhibitors to lower tumor pressure, enzymes that degrade matrix, or immune checkpoint blockers for immunogenic synergy). A forward-looking R&D strategy will build a combination platform around an ADC, enabling a tailored approach depending on tumor characteristics (for a fibrotic tumor use a FAK inhibitor, for an immune-cold tumor perhaps an immune agonist, etc.).
- Intellectual Property and Differentiation: Combining two agents can raise questions about intellectual property (IP) and exclusivity. Companies should seek to generate IP on specific combinations (for example, method-of-use patents for the use of FAK inhibitors with ADCs in certain indications). The recent research from InxMed indicates patent filings around this concept pubmed.ncbi.nlm.nih.gov. Securing IP will ensure that if the combo proves effective, the company has protected its ability to market that regimen exclusively (or to negotiate terms if others want to use the idea). From a differentiation standpoint, developing a companion diagnostic (e.g., a test that measures FAK activity or stromal biomarkers in tumors) could further cement a company’s leadership – it positions the company not just as selling drugs but offering a personalized solution for high-stroma cancers.
- Resource Allocation and Organizational Alignment: Pursuing combination therapy development requires coordination between different teams – those who typically work on biologics/ADCs and those who work on small molecules. Senior leadership should ensure that internal teams are aligned and possibly integrated for such projects, as timing of development needs to be synchronized (chemistry, manufacturing, and controls for a small molecule vs. biologic manufacturing are different beasts). Budget planning should account for combination trials which tend to be more complex (two drugs, perhaps separate sourcing or blinding). However, the upside is high: a successful ADC+FAK inhibitor combination could lead to multiple new indications for the ADC and a rejuvenation of interest in the FAK inhibitor program. Leaders should weigh this against the investment – in many cases, combining existing assets could be far more cost-effective and faster than developing an entirely new drug for the same indications.
In implementing these strategic actions, it’s crucial to remain data-driven. Continuously monitor emerging results from competitors or academia working on similar approaches. For instance, if another group demonstrates that a different TME modulator (say, a CXCR4 inhibitor) also boosts ADC efficacy, be prepared to compare and perhaps combine approaches (could FAK inhibition plus another stromal modulator yield even more benefit? These questions will arise.). The field of ADCs is evolving rapidly, and maintaining a scientific edge will require agility and openness to combination innovation.
Conclusion and Outlook
The therapeutic strategy of combining FAK inhibitors with antibody–drug conjugates represents a convergence of two powerful oncology approaches: precision targeting of cancer cells and remodeling of the tumor microenvironment. This combined approach directly addresses a long-standing challenge in solid tumor therapy – the physical and biological barriers that limit drug delivery. By breaking down tumor defenses and enabling deeper ADC penetration, FAK inhibition has the potential to unlock the full efficacy of ADCs in patients who currently derive limited benefit from these agents pmc.ncbi.nlm.nih.govdelta.larvol.com. The preclinical evidence is compelling, showing dramatic improvements in anti-tumor response when these therapies are used together pmc.ncbi.nlm.nih.gov. Early expert commentary and company commitments suggest that the oncology community recognizes this potential and is moving swiftly to translate it into clinical reality en.inxmed.comen.inxmed.com.
From a strategic leadership perspective, the coming 1-3 years will be critical. We will likely see first-in-human trial readouts of FAK+ADC combinations. If they confirm even a fraction of the preclinical synergy, it could herald a new paradigm in oncology treatment: one in which drug efficacy is no longer viewed in isolation, but in the context of a modifiable tumor ecosystem. In practical terms, oncologists of the future might prescribe an ADC alongside a tumor-penetration enhancer (like a FAK inhibitor) as a standard regimen for certain cancers, much as we now combine chemo with radiotherapy or immunotherapy for greater effect. For R&D leaders, this means that developing combination know-how is as important as developing the drugs themselves. It calls for a shift in mindset – success may come not just from the next “miracle drug” but from smartly combining two good drugs to achieve superior results.
In conclusion, the strategy of FAK inhibition combined with ADCs holds considerable promise to improve patient outcomes by overcoming the stromal barriers to treatment. It exemplifies the kind of innovative, cross-disciplinary thinking that drives progress in oncology: uniting cell biology (understanding CAFs and FAK signaling) with advanced drug engineering (ADCs) and clinical insight. As always, careful clinical investigation will determine how this strategy performs in humans. But the strong rationale and supportive data to date warrant enthusiasm. Senior scientific leaders should keep a close eye on developments in this area – and more proactively, consider positioning their organizations at the forefront of this movement. By investing in and championing approaches like FAK+ADC combinations, we can aim to expand the reach of precision therapies into the most treatment-resistant tumors, ultimately delivering stronger anti-cancer responses and hope for patients who need new solutions.
Sources: Recent research and expert commentary have been drawn from high-impact studies and publications, including iScience (Cell Press) pmc.ncbi.nlm.nih.gov, Frontiers in Pharmacology delta.larvol.com, and presentations at AACR and World ADC conferences delta.larvol.com, as well as industry press releases en.inxmed.comen.inxmed.com. These sources provide the scientific and strategic foundation for the points discussed in this report.


