Accelerating Antibody Discovery with Single B Cell Technology: A High-Throughput Platform by Biointron
In the rapidly advancing field of therapeutic antibody development, speed, specificity, and diversity remain crucial for successful candidate selection. Traditional methods such as hybridoma and phage display technologies, while foundational, often fall short in delivering the throughput and affinity required for next-generation biologics. To address this gap, Biointron has developed a robust, high-throughput single B cell antibody discovery platform, offering a fast and efficient pathway from immunization to lead identification.
A Next-Generation Antibody Discovery Platform
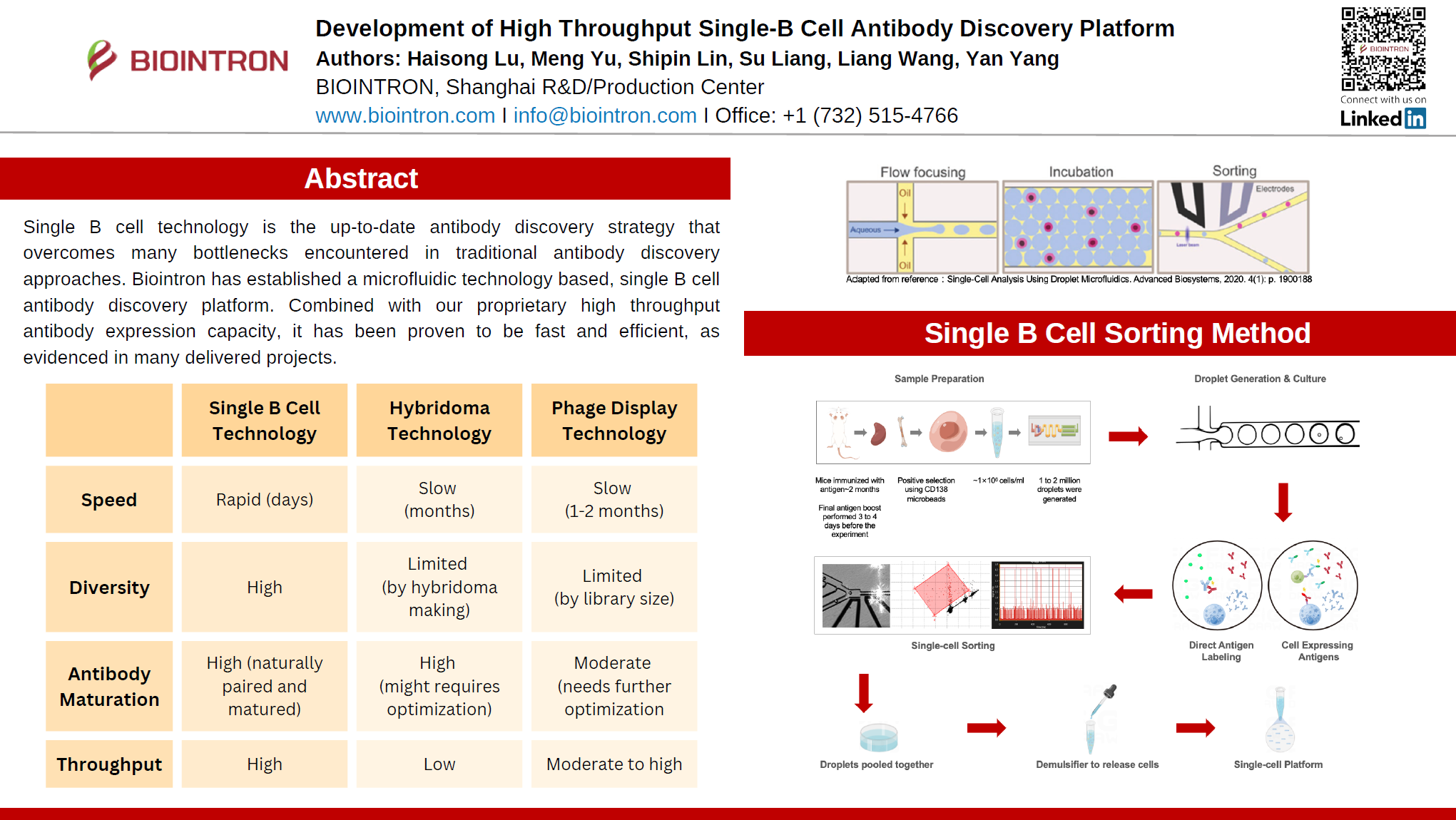
Biointron’s platform leverages microfluidic droplet-based single B cell encapsulation. Each droplet contains an individual plasma B cell, where secreted antibodies are captured via fluorescently labeled antigens. Antigen-specific cells are rapidly identified through FRET-based fluorescence emission at 620 nm, allowing for precise selection of antibody-secreting cells.
Compared to traditional platforms:
-
Speed: Antibodies can be discovered within days, significantly faster than hybridoma (months) or phage display (1–2 months).
-
Diversity: Naturally paired, in vivo matured antibodies maintain native CDR diversity, outperforming the limited diversity of hybridoma and phage display libraries.
-
Throughput: The microfluidic approach supports screening of thousands of B cells, achieving high hit rates and rapid progress.
Case Study 1: Tumor-Associated Antigens
In a representative project, Biointron's single B cell platform was used to generate antibodies targeting a tumor-associated soluble antigen. Initial ELISA screening yielded 62 antigen-binding candidates. Of these, 22 were selected for surface plasmon resonance (SPR) affinity analysis, all displaying high affinity in the 0.1–1 nM range. Several candidates demonstrated superior kinetic profiles compared to benchmark controls.
Case Study 2: PD-1-Specific Antibodies
In a second application, 59 antibodies binding to PD-1 were identified and characterized. Using SPR, their affinities were ranked, with nearly all candidates showing KD values between 0.1–1 nM. Epitope binning divided the antibodies into four distinct groups, confirming the platform's ability to generate diverse antibodies against different PD-1 epitopes. Benchmark controls included nivolumab and pembrolizumab biosimilars, with several novel antibodies exhibiting distinct binding profiles.
Toward Hard-to-Target Proteins
Biointron's current focus extends to the development of detection schemes for transmembrane proteins, particularly G protein–coupled receptors (GPCRs). These targets are notoriously difficult for traditional antibody generation methods, but the single B cell platform is poised to address this challenge due to its flexibility and robustness.
Conclusion
With the ability to deliver diverse, high-affinity antibodies in just 2–3 months, Biointron’s single B cell antibody discovery platform represents a transformative advancement for therapeutic antibody development. By combining state-of-the-art microfluidics with high-throughput screening and expression capabilities, the platform opens new avenues for targeting both soluble and membrane-bound proteins, including those previously considered intractable.
For more information, visit www.biointron.com or contact info@biointron.com.